Testing Donor Tissue for M. tuberculosis Complex
Donor tissue may have a detectable presence of Mycobacterium tuberculosis complex (MTBC) or nontuberculous mycobacterium (NTM), which may be transmitted to patients. The FDA has recently issued new guidance recommending the screening of donor tissue for Mtb infection or contamination before use to reduce the risk of transmission.
National Jewish Health Advanced Diagnostic Laboratories offers customized extraction and detection testing for donor tissue samples to help determine sample safety.
About Donor Tissue Sample Testing
Advanced Diagnostic Laboratories performs mycobacterial culture on solid and liquid media. Because of the long turnaround of culture, the lab has also developed a unique semi-automated, rapid turnaround PCR assay for MTBC detection from bone matrix samples in order to accurately pre-screen donor tissue samples. These options can be used in tandem as a two-step method for rapid and accurate testing of donor samples.
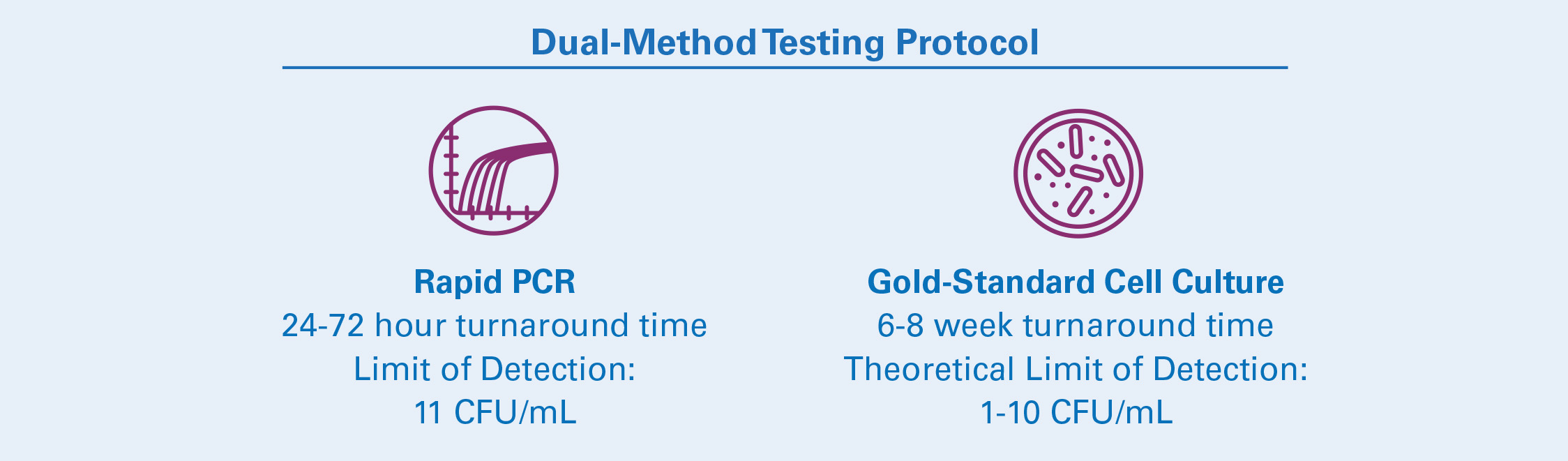
- All potential MTBC samples must be handled in a BSL3 laboratory.
- FDA guidelines recommend screening donor tissue before use. National Jewish Health offers dual-method testing to ensure the highest accuracy for detection of potential MTBC.
- Evaluation for suitability to detect inhibition recommended.
- Tests are performed as Research Use Only (RUO) for donor tissue pre-screening.
Our Expertise
National Jewish Health Advanced Diagnostic Laboratories has extensive experience diagnosing tuberculosis (TB) and mycobacterial diseases, dating back to 1899. Our Mycobacteriology Laboratory is recognized worldwide as a leading diagnostic laboratory for MTBC and NTM.
How to Order
Call 303.398.1669 or email ADxSales@njhealth.org to speak with our business development team about how we can partner to meet your tissue testing needs.
